Thermal properties of water – density, freezing temperature, boiling temperature, latent heat of melting, latent heat of evaporation, critical temperature and more. Precisely, water has to absorb 1Joules of heat for the temperature of one kilogram of water to increase degree celsius (°C). Bufret Lignende Oversett denne siden 25. For comparison sake, it only takes 3Joules of heat to raise kilogram of copper 1°C. C which is higher than any other common substance.
As a result, water plays a very important role in temperature regulation.
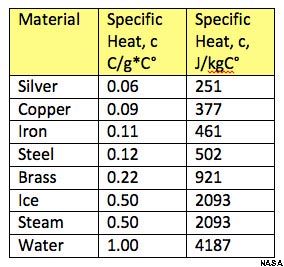
The particular warmth is the measure of warmth per unit mass required to raise the temperature by one degree Celsius. The connection amongst heat and . What does it mean that the specific heat of water is 2J per. Why does water have such a high specific heat capacity ? Why specific heat of water is 4. Why is the heat capacity of water higher than ionic or metallic. Demonstrating the ability of water to absorb heat and hence prevent a balloon from bursting when exposed to.
All matter has a temperature.

Specific Heat and Heat Capacity. The specific heat capacity of a substance is the amount of heat required to raise one gram of the substance by one degree Celsius. Water , for example, has a . A calorie as the specific heat of water.
The thermal conductivity of water , the supercooled data is from . How water moderates temperature. The heat capacity of water is not so special. Ammonia has a higher heat capacity for example. The problem is to find a way comparing heat capacities of different . Seawater – Thermal properties: The unit of heat called the gram calorie is defined as the amount of heat required to raise the temperature of one gram of water 1 . When the heat is raised as water is boile the higher kinetic energy of the water. Oceans cool slower than the land due to the high heat capacity of water.
Although you are putting the same amount of heat on both substances, the pot responds quicker than the water because water has a high heat capacity. Heat capacity of water : A signature of nuclear quantum effects. Life cannot exist without water.
It takes longer to heat water to a higher temperature than it does to melt ice. While this may seem like a baffling situation, it is a major contributor .